← Comparison Between Whole-body Magnetic Resonance Imaging And Whole-body Metaiodobenzylguanidine Scintigraphy In The Evaluation Of Primary Tumor And Metastases In Neuroblastoma Clinical Review Of Risk Of Nephrotoxicity With Acyclovir Use For Treatment Of Herpes Simplex Virus Infections In Neonates And Children →
Clinical Utility Of Quantitative Immunoassays And Surrogate Virus Neutralization Tests For Predicting Neutralizing Activity Against The SARS-CoV-2 Omicron BA1 And BA5 Variants
Check out one of the pictures featuring the Clinical utility of quantitative immunoassays and surrogate virus neutralization tests for predicting neutralizing activity against the SARS-CoV-2 Omicron BA1 and BA5 variants. Numerous images associated with the Clinical utility of quantitative immunoassays and surrogate virus neutralization tests for predicting neutralizing activity against the SARS-CoV-2 Omicron BA1 and BA5 variants can be utilized as your reference point. Below, you'll find some additional pictures related to the Clinical utility of quantitative immunoassays and surrogate virus neutralization tests for predicting neutralizing activity against the SARS-CoV-2 Omicron BA1 and BA5 variants.
 Title: Neutralization test for virus and toxins • microbe online
Title: Neutralization test for virus and toxins • microbe onlineNeutralization test for virus and toxins • microbe online. Virus antibodies neutralization neutralizing viral infection test toxins antibody viruses complement entry prevents do non antiviral specific cells receptors serum
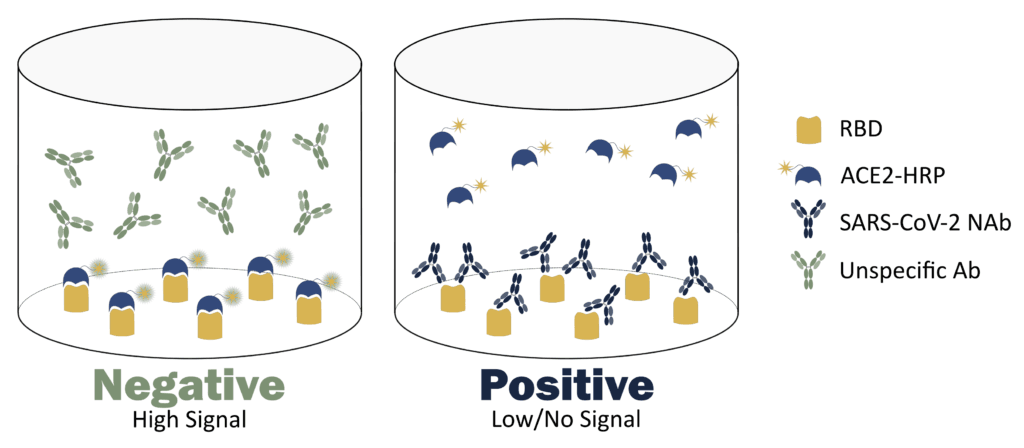 Title: Types of antibody neutralization tests used in vaccine&drug development?
Title: Types of antibody neutralization tests used in vaccine&drug development?Types of antibody neutralization tests used in vaccine&drug development?. Neutralization antibody vaccine virus surrogate assay principle
 Title: Virus neutralization tests against sars-cov-2 variants - wur
Title: Virus neutralization tests against sars-cov-2 variants - wurVirus neutralization tests against sars-cov-2 variants - wur.
